 |
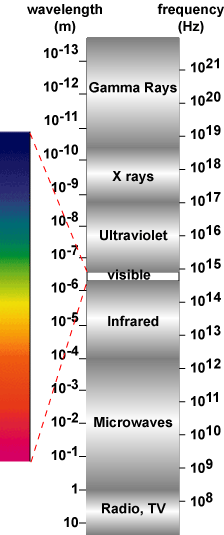
| The electromagnetic spectrum. |
The light that we see everyday is only a fraction of the total energy emitted by the sun incident on the earth. Sunlight is a form of "electromagnetic radiation" and the visible light that we see is a small subset of the electromagnetic spectrum shown at the right.
The electromagnetic spectrum describes light as a wave which has a particular wavelength. The description of light as a wave first gained acceptance in the early 1800's when experiments by Thomas Young, François Arago, and Augustin Jean Fresnel showed interference effects in light beams, indicating that light is made of waves. By the late 1860's light was viewed as part of the electromagnetic spectrum. However, in the late 1800's a problem with the wave-based view of light became apparent when experiments measuring the spectrum of wavelengths from heated objects could not be explained using the wave-based equations of light. This discrepancy was resolved by the works of 1 in 1900, and 2 in 1905. Planck proposed that the total energy of light is made up of indistinguishable energy elements, or a quanta of energy. Einstein, while examining the photoelectric effect (the release of electrons from certain metals and semiconductors when struck by light), correctly distinguished the values of these quantum energy elements. For their work in this area Planck and Einstein won the Nobel prize for physics in 1918 and 1921, respectively and based on this work, light may be viewed as consisting of "packets" or particles of energy, called photons..
Today, quantum-mechanics explains both the observations of the wave nature and the particle nature of light. In quantum mechanics, a photon, like all other quantum-mechanical particles such as electrons, protons etc, is most accurately pictured as a "wave-packet". A wave packet is defined as a collection of waves which may interact in such a way that the wave-packet may either appear spatially localized (in a similar fashion as a square wave which results from the addition of an infinite number of sine waves), or may alternately appear simply as a wave. In the cases where the wave-packet is spatially localized, it acts as a particle. Therefore, depending on the situation, a photon may appear as either a wave or as a particle and this concept is called "wave-particle duality".
A complete physical description of the properties of light requires a quantum-mechanical analysis of light, since light is a type of quantum-mechanical particle called a photon. For photovoltaic applications, this level of detail is seldom required and therefore only a few sentences on the quantum nature of light are given here. However, in some situations (fortunately, rarely encountered in PV systems), light may behave in a manner which seems to defy common sense, based on the simple explanations given here. The term "common sense" refers to our own observations and cannot be relied on to observe the quantum-mechanical effects because these occur under conditions outside the range of human observation. For further information on the modern interpretation of light please refer to 3. A wave-packet, or photon is pictured as used in PVCDROM below.
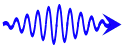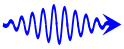 High energy photon for blue light.
High energy photon for blue light.
 Lower energy photon for red light.
Lower energy photon for red light.
 Low energy photon for infrared light. (should be invisible)
Low energy photon for infrared light. (should be invisible)
There are several key characteristics of the incident solar energy which are critical in determining how the incident sunlight interacts with a photovoltaic converter or any other object. The important characteristics of the incident solar energy are:
- the spectral content of the incident light;
- the radiant power density from the sun;
- the angle at which the incident solar radiation strikes a photovoltaic module; and
- the radiant energy from the sun throughout a year or day for a particular surface.
By the end of this chapter you should be familiar with the above four concepts..
- 1. , “Distribution of energy in the normal spectrum”, Verhandlungen der Deutschen Physikalischen Gesellschaft, vol. 2, pp. 237-245, 1900.
- 2. , “Generation and transformation of light”, Annalen der Physik, vol. 17, 1905.
- 3. , QED : The Strange Theory of Light and Matter. 1985.
- Log in or register to post comments
- 3 comment(s)
 Español
Español 한국어
한국어 简体中文
简体中文 Bahasa Indonesia
Bahasa Indonesia
